Why You Shouldn’t Spend Money on BCAA’s (Free Form Branched-Chain Amino Acids)
BCAA’s
Branched-Chain Amino Acid, or BCAA, supplements are one of the most lucrative products on the market today. Their sales bring in millions of dollars per year for the supplement industry. Proponents (read: people who profit off the sale of BCAAs) claim a broad range of benefits from recovery, to anti-catabolism, to performance, to muscle building. But do they work in a practical sense? Skip to the Conclusion section if you are late for the gym!

First, a little background
BCAAs are found in all complete food protein sources. They are necessary for health, and when consumed in a food-based diet are ingested with a myriad of other beneficial substances that exert positive effects on the body. This article itself deals with the further supplementation of Free Form Branched-Chain Amino Acids rather than food sources.
For the purposes of this article we will only be discussing the proteinogenic branched-chain amino acids of which there are three. Leucine, isoleucine, and valine. These constitute 3 of the 9 essential amino acids for humans, accounting for 35% of the essential amino acids in muscle protein.1 BCAAs are extremely important metabolically as they promote signaling pathways, metabolism of glucose, and protein synthesis and turnover.2,3
BCAAs also share the same transport protein across the blood brain barrier with Tryptophan, Tyrosine, and Phenylalanine. In the brain, BCAAs may play a role in synthesis of neurotransmitters, production of energy, and protein synthesis.2
Bored yet? Just wait. Let’s get to some meat (pun intended).
The beginning
The original studies that started the hysteria back in the late 90s to mid 2000s for these products were based on murine models where rats were essentially starved then made to tread water. Lo and behold, a starving rat that was given a BCAA cocktail was able to tread water longer. Therefore, the thought process went, BCAAs taken around or during the workout must be providing aminos required for increased time to exhaustion, recovery, and performance benefits!
Later research suggests this effect was due to a sub-optimal protein intake and the fact that BCAAs themselves contain calories providing energy. Much to the surprise of people who purchase BCAA supplements touting 0 calories, they do contain around 4.65 cals/g.4 Due to FDA labeling loopholes, free form amino acids are not required to list calorie content and therefore opt to show their products as zero calorie.
Another example from this time period (late 90s) was a study based on a group of wrestlers weighing 150lbs and only taking in around 80g of protein (hardly enough). The participants were given a whopping 52g of BCAAs/day.19 The positive results from these studies were not able to be replicated once adequate protein was being administered to the tested athletes.
The second wave of hype for BCAAs came based on Muscle Protein Synthesis (MPS) stimulation research. And the practice of spacing supplemental BCAAs out between meals to maximize MPS was born. The idea was that if you could maximize the “on” rate of muscle protein production machinery that would be a good thing, right? This was ultimately proven disadvantageous for several reasons which we will discuss in depth later.
As a point of additional background: Leucine itself is likely the most important trigger for MPS. An analogy would be that Leucine is the spark plug in a car. The problem being, without the other essential amino acids (not just the BCAAs), the body does not have enough substrate to keep the car running. The essential amino acids in this (admittedly poor) analogy would be the gasoline. MPS may potentially enter a refractory state if it is triggered too frequently. This can prevent maximal benefit of the “machinery”. Based on current research, eating no sooner than every ~3 hours or so may be the optimal time period to prevent entering a refractory state (and if hypertrophy is your main goal). Meal timing is a topic for another day, however.
The now
The breadth of research on BCAAs has essentially exploded over the past two decades. In almost all applications, given an adequate daily protein intake (even when training fasted), BCAA supplements appear to be at best an expensive water flavouring, and at worst potentially detrimental through various mechanisms. This is vital to understand when evaluating BCAA research. Studies showing benefits inevitably have not controlled for protein intake. Let’s delve into the conclusions of well controlled studies (adequate daily protein intake, blinded, minimal confounding variables, etc).
- Null effects:
- “However, results from the present study suggest that ingesting BCAAs alone, without the other EAA, provides limited substrate for protein synthesis in exercised muscles. Thus, the overall response of MPS is not maximized. Instead, the limited availability of EAA likely explains the qualitative difference in magnitude of the MPS response to ingestion of BCAAs alone and ingestion of similar amounts of BCAAs as part of intact whey protein (Churchward-Venne et al., 2012, 2014; Witard et al., 2014).”7
- Supplementing Leucine or free form BCAAs on top of a sufficient dietary protein intake does not appear to yield beneficial results. As little as 1.1g protein/kg bodyweight per day appears to render additional amino supplementation pointless. It did not have a beneficial effect on muscle strength, physical functioning, aerobic capacity, or cardiometabolic health.31
- “We conclude that the claim that consumption of dietary BCAAs stimulates muscle protein synthesis or produces an anabolic response in human subjects is unwarranted [in the post-absorptive state].”8
- “The results of this study showed that BCAAs supplementation has no effect on the reduction of lactate and ammonium indices as indicators of fatigue.”29
- “These results provide new insights into the regulation of MPS by demonstrating that leucine and mTOR signaling alone are not responsible for the muscle anabolic effect of protein ingestion during physiological hyperinsulinaemia, most probably because they fail to signal to eIF2α to initiate translation and/or additional amino acids are needed to sustain translation.”15 Another potential example of the need for substrate (the rest of the EAAs) for optimal MPS.
- “The findings of this study suggest that the acute ingestion of an Amino Acid/Electrolyte beverage during upper body resistance exercise does not alter acute muscle thickness, performance, perceived soreness and weakness, or markers of muscle damage.”16
- When protein intake has been accounted for, the research shows that supplementing with additional leucine does not provide any extra benefit in terms of muscle size or strength compared to placebo.20
- When even moderate (minimal, in my opinion) levels of protein are consumed in the daily diet, the attenuation of muscular performance decrements or corresponding plasma CK levels are negligible. This suggests that recovery is not improved via BCAAs when the athlete in question has an adequate protein diet.24
- “When combined with heavy resistance training for 8 weeks, supplementation with 9 g/day of BCAA 30 min before and after exercise had no preferential effects on body composition and muscle performance.”25
- Negative effects:
- “As noted in a recent review by Morton et al., there is a paucity of evidence supporting a beneficial effect for BCAA supplementation in promoting increases in muscle protein synthesis or lean mass, and in fact there might be a detrimental impact given that the AAs appear to antagonize each other in terms of transport both into circulation and likely into the muscle.”5
- “Both elevated fatty liver index (FLI) and elevated plasma BCAA levels are associated with risk of incident type 2 diabetes (T2D). The association of non-alcoholic fatty liver disease (NAFLD) with T2D development seems partly mediated by elevated BCAAs.” I do not wish to be alarmist in this sense. But it would be prudent, especially for those that are pre-diabetic, to abstain from pushing BCAA supplements.30
- “Despite the popularity of BCAA supplements we find shockingly little evidence for their efficacy in promoting MPS or lean mass gains and would advise the use of intact proteins as opposed to a purified combination of BCAA”6
- An extensive search of the literature has revealed no studies in human subjects in which the response of muscle protein synthesis to orally-ingested BCAAs alone was quantified, and only two studies in which the effect of intravenously infused BCAAs alone was assessed. Both of these intravenous infusion studies found that BCAAs decreased muscle protein synthesis [in the post-absorptive state] as well as increased protein breakdown, meaning a decrease in muscle protein turnover. The catabolic state in which the rate of muscle protein breakdown exceeded the rate of muscle protein synthesis persisted during BCAA infusion.8
- “When all evidence and theory is considered together, it is reasonable to conclude that there is no credible evidence that ingestion of a dietary supplement of BCAAs alone results in a physiologically-significant stimulation of muscle protein. In fact, available evidence indicates that BCAAs actually decrease muscle protein synthesis and increase protein breakdown [in the post-absorptive state]. All EAAs must be available in abundance for increased anabolic signaling to translate to accelerated muscle protein synthesis”.8
- “The effect of consuming BCAA between protein-rich meals would be negligible due to the ‘muscle full’ effect – if protein has been consumed recently (~3h), availability of leucine and EAAs do not further induce MPS after the transient peak and may send MPS into a refractory state.”11
- Holecek et al’s study (unfortunately a murine/rodent model and therefore questionable relation to human physiology) suggests that an overabundance of BCAAs could trigger an over-expression of the BCAA degrading enzyme BCKA dehydrogenase and the subsequent conversion of BCAAs to BCAA keto acids and/or eventually alanine or glutamine which are then used/abused as an energy source by the liver.14
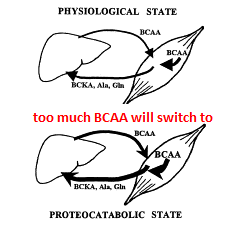
Photo adapted from Moussa
- Another study suggests competitive gut absorption caused by overabundance of certain aminos. “Active absorption of amino acids was rapid at low concentrations and depressed at higher concentrations.”17 Added on top of a whole protein source, this potentially causes competitive absorption limiting muscle growth. In other words, taking all 3 BCAAs together lowers Leucine’s muscle building signal (as they tend to interfere with each others absorption) especially if you add a large amount to another protein source.
The muscle full effect is currently theoretical, and requires more research to identify specifics.
Other thoughts
BCAAs are often touted as beneficial for being anti-catabolic and are recommended peri-workout for this application. As the current research shows us, they don’t appear to be beneficial if you have a decent diet, even when fasted. Supposed losses of muscle that BCAAs (and some metabolites such as HMB) are meant to attenuate from common resistance training in humans are trivial or non-existent to begin with.26,27,28 BCAAs are commonly utilized (even after an overnight fast, intra-workout, and in 16:8 IF protocols) to solve a problem that does not exist. Regardless, reducing muscle breakdown peri-workout is a poor choice (and unneeded) for many reasons. Least of which, that muscle breakdown due to resistance training is beneficial.
- “We conclude that muscle hypertrophy is the result of accumulated intermittent changes in MyoPS post-RE in RT, which coincides with progressive attenuation of muscle damage.”9
- “Hypertrophic remodeling is most active during the early stages of Resistance Exercise Training, reflecting longer-term MPS.”10 The more a person is trained, the less you would want to dampen that muscle breakdown effect as they have a decreased hypertrophic remodeling response.
Burn victims, in the past, were also given BCAAs as an adjunct treatment. However, a 2006 paper suggests that the concept of nutrition supplemented with BCAAs for burns, trauma, and sepsis should be abandoned for a more promising leucine (and glutamine)-only-supplemented nutrition that requires further evaluation.12
“Certain studies suggested a possible link between a high incidence of amyotrophic lateral sclerosis (ALS) among professional American football players and Italian soccer players, and certain sports supplements including BCAAs.”13 Although I am not overly convinced by this research, I have included it for completeness sake.
Tipton states: When a group of male subjects were given 5.6g of BCAAs following a session of lifting weights – the equivalent to 20g of whey – the resulting protein synthesis response was only about 22%, or about half, of what would be achieved with the equivalent dose of whey.22,23
Many BCAA proponents will point to a 2009 Stoppani study as proof that BCAAs must work!21 Unfortunately, diet is not controlled, calories and protein intake are neither controlled nor accounted for, the study’s write-up is vague and non-specific, no supplemental data is present, and it does not appear to have gone through the peer-review process.
Conclusion
I would consider supplementing with a full EAA product (rather than solely BCAAs) in chronic renal failure or urea cycle disorders, or as a supplement to whole dietary protein sources in the elderly.18 In the case of restrictive vegan/vegetarian diets, supplementation with L-Leucine alone is likely adequate.
Lastly, BCAAs/EAAs may provide some benefits, particularly when paired with carbohydrates, in ultra endurance training or in single-day multi-event scenarios (such as a Crossfit competition) when an athlete is unable to consume a meal or protein shake.
I believe Professor Stuart Phillips, PhD said it best with “BCAA are quite possibly the biggest rip off, perhaps second to fat burners, in the entire supplement industry. And remember the plural of anecdotes that they work is still NOT data or (in any way) proof that BCAAs ‘work’”.
Verdict: save your money and purchase a dairy-sourced protein powder or tasty high protein food (or a Maui Athletics t-shirt)!

Citations:
- Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA (June 2004). “Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise”. The Journal of Nutrition. 134 (6 Suppl): 1583S–1587S. PMID 15173434
- Monirujjaman M (2014). “Metabolic and Physiological Roles of Branched-Chain Amino Acids”. Advances in Molecular Biology. 2014: 1–6. doi:10.1155/2014/364976. Retrieved 26 November 2016.
- Babchia N, Calipel A, Mouriaux F, Faussat AM, Mascarelli F (January 2010). “The PI3K/Akt and mTOR/P70S6K signaling pathways in human uveal melanoma cells: interaction with B-Raf/ERK”. Investigative Ophthalmology & Visual Science. 51 (1): 421–9. doi:10.1167/iovs.09-3974. PMID 19661225
- doi.org/10.1093/ajcn/52.5.770
- J Int Soc Sports Nutr. 2016 May 11;13:21. The data do not seem to support a benefit to BCAA supplementation during periods of caloric restriction. Dieter BP1, Schoenfeld BJ2, Aragon AA3.
- Front Physiol. 2015 Sep 3;6:245. doi: 10.3389/fphys.2015.00245. eCollection 2015.Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy.Morton RW1, McGlory C1, Phillips SM1.
- Jackman SR, Witard OC, Philp A, Wallis GA, Baar K and Tipton KD (2017) Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Front. Physiol. 8:390. doi: 10.3389/fphys.2017.00390
- Wolfe, Branched-chain amino acids and muscle protein synthesis in humans: myth or reality? Journal of the International Society of Sports Nutrition (2017) 14:30 DOI 10.1186/s12970-017-0184-9
- https://www.ncbi.nlm.nih.gov/pubmed/27219125
- https://www.ncbi.nlm.nih.gov/pubmed/26169934
- https://www.ncbi.nlm.nih.gov/pubmed/20844073
- De Bandt JP, Cynober L (January 2006). “Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis”. The Journal of Nutrition. 1 Suppl. 136 (1 Suppl): 308S–13S. PMID 16365104.
- Manuel M, Heckman CJ (March 2011). “Stronger is not always better: could a bodybuilding dietary supplement lead to ALS?”. Experimental Neurology. 228 (1): 5–8. doi:10.1016/j.expneurol.2010.12.007. PMC 3049458 . PMID 21167830
- Holeček, Milan. “The BCAA–BCKA cycle: its relation to alanine and glutamine synthesis and protein balance.” Nutrition 17.1 (2001): 70.
- https://physoc.onlinelibrary.wiley.com/doi/abs/10.1113/JP276504
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5968991/
- https://www.ncbi.nlm.nih.gov/pubmed/5965263
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5934885/
- https://www.ncbi.nlm.nih.gov/pubmed/9059905
- Aguiar AF, Grala AP, da Silva RA, et al. Free leucine supplementation during an 8-week resistance training program does not increase muscle mass and strength in untrained young adult subjects. Amino Acids. 2017;49(7):1255-1262. doi:10.1007/s00726-017-2427-0. https://www.ncbi.nlm.nih.gov/pubmed/28444456
- Stoppani, Jim, et al; “Consuming a supplement containing branched-chain amino acids during a resistance-training program increases lean mass, muscle strength and fat loss”; Journal of the International Society of Sports Nutrition; 2009; 6(Suppl 1):P1; https://jissn.biomedcentral.com/articles/10.1186/1550-2783-6-S1-P1
- https://www.ncbi.nlm.nih.gov/pubmed/28638350
- https://www.ncbi.nlm.nih.gov/pubmed/24257722
- https://www.mdpi.com/2072-6643/10/10/1389
- https://www.ncbi.nlm.nih.gov/pubmed/24620007
- https://www.ncbi.nlm.nih.gov/pubmed/9252485
- https://www.ncbi.nlm.nih.gov/pubmed/12489923
- https://www.ncbi.nlm.nih.gov/pubmed/24791918
- https://www.ncbi.nlm.nih.gov/pubmed/30792920
- https://www.mdpi.com/2072-6643/11/3/705
- https://www.frontiersin.org/articles/10.3389/fphys.2019.00445/full